

Prof Alain Gibaud
Prof Alain Gibaud, a distinguished Emeritus Professor at Le Mans Université, gave an enlightening presentation on X-ray nanoscopy of calcium carbonate (CaCO3). This is a key material in daily life. The presentation was part of a webinar series hosted by Prof Malik Maaza, the UNESCO-UNISA Africa Chair in Nanoscience and Nanotechnology (U2ACN2) at the College of Graduate Studies.
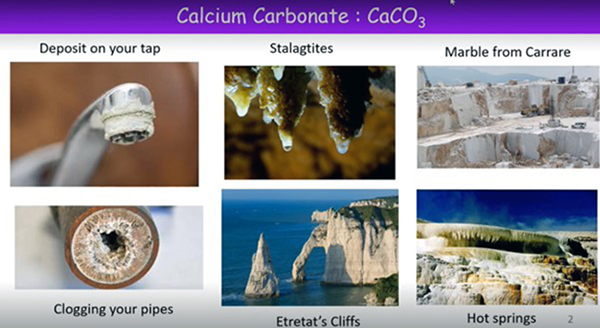
Calcium carbonate is a material that is found in many natural species. For example, in South Africa, calcium carbonate can be seen in shells such as oyster shells, coccolithophores, pearls, abalone, nautilus and coral, eggshells and snail shells. Calcium carbonate is a compound used extensively in the chemical industry, environmental industry, material filter, biomedical and food industries. In addition, nanoparticles made from calcium carbonate in antacid tablets are new weapons in the war against cancer.
Amorphous calcium carbonate is the easiest polymorph to form from a kinetics point of view. Ultimately this polymorph transforms into more stable forms such as vaterite, aragonite and calcite. X-ray powder diffraction and scanning electron microscopy commonly identify the specific polymorph formed.
The analysis of precipitated calcium carbonate particles by coherent X-ray diffraction imaging (CXDI) at the ID10 beamline of the European Synchrotron Radiation Facility (ERSF) provides evidence that precipitation without polystyrene sulfonate (PSS) results in vaterite that progressively transforms into more stable calcite when kept in water. Precipitation with PSS yields spherical particles in the early stages of ageing in hot water at 75 °C. PSS was found to stabilise vaterite and delay the precipitation. After 24 hours of residence in the water, hollow-core particles are formed. The core gradually dissolves, and vaterite particles recrystallise on the shell. The crystals are mainly oriented with their c-axis along the meridians of the particle. The frustration of their dipolar moments yields a depleted region at the poles.
Concluding his presentation, Gibaud mentioned that CXDI is a unique technique used in imagining three-dimensional (3D) objects of small size (S<8µm) with a resolution close to 30 nm. It enables obtaining more information on the inner structure of materials. In addition, it can be used to monitor physical parameters such as the time-dependent evolution of particles. Segmenting also enables extracting specific particle elements, such as tiny crystals, and exploring their properties.
To watch the recordings of the webinar research series, click here.
* By Hanli Wolhuter, Communication and Marketing Specialist and Musa Buthelezi, Intern, College of Graduate Studies
Publish date: 2022-12-07 00:00:00.0